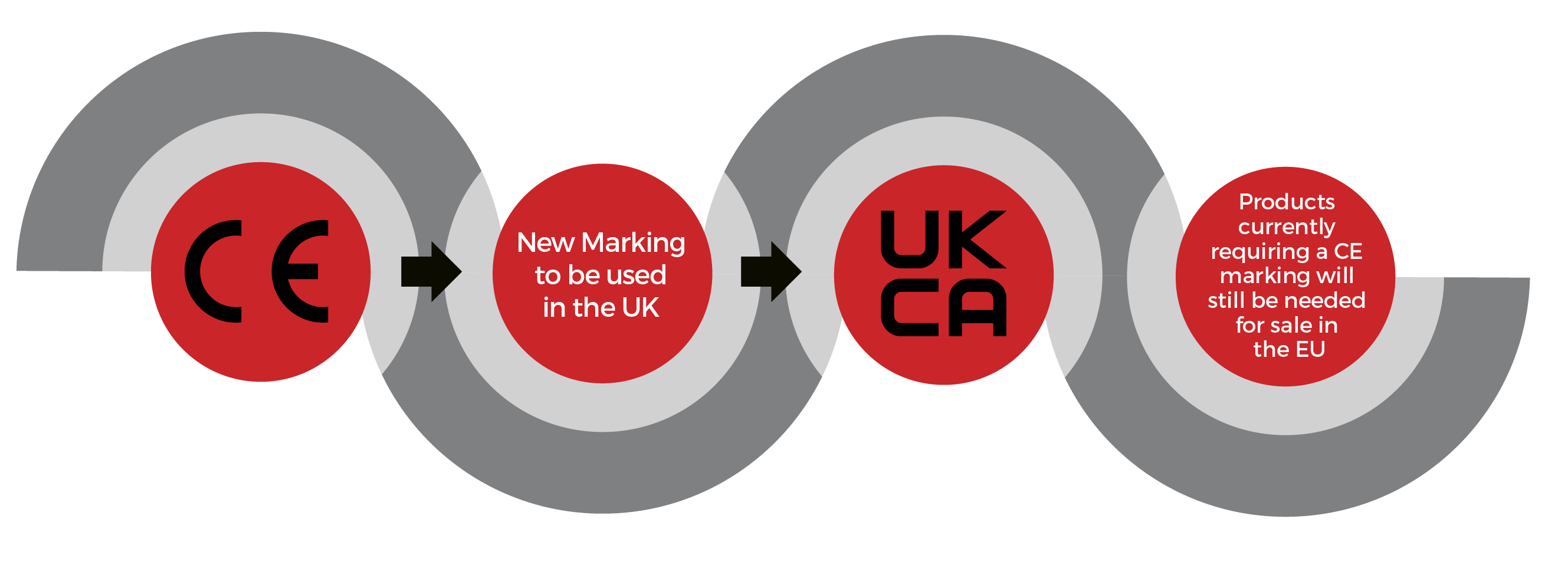
Ukca Marking Medical Devices Deadline . medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. You can continue to use the. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. Ukca mark will be mandatory for. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. the deadline for when businesses need to use the ukca mark has been extended. but significant changes are planned. What will the new uk regulation look like? ukca marking deadline for medical devices & ivds. what’s the latest timetable for implementing ukca markings for medical devices? a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements.
from www.productapprovals.co.uk
deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. Ukca mark will be mandatory for. ukca marking deadline for medical devices & ivds. the deadline for when businesses need to use the ukca mark has been extended. What will the new uk regulation look like? medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. You can continue to use the. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. but significant changes are planned.
UKCA marking UK
Ukca Marking Medical Devices Deadline what’s the latest timetable for implementing ukca markings for medical devices? The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. What will the new uk regulation look like? medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. You can continue to use the. Ukca mark will be mandatory for. what’s the latest timetable for implementing ukca markings for medical devices? ukca marking deadline for medical devices & ivds. but significant changes are planned. the deadline for when businesses need to use the ukca mark has been extended.
From www.greensofttech.com
UKCA Mark Deadline Extended 2 Yrs Environmental Compliance GreenSoft Ukca Marking Medical Devices Deadline medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. ukca marking deadline for medical devices & ivds. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. You can continue to use the. what’s. Ukca Marking Medical Devices Deadline.
From blog.clevercompliance.io
UKCA Marking vs CE Marking FAQ General Product Compliance Ukca Marking Medical Devices Deadline what’s the latest timetable for implementing ukca markings for medical devices? You can continue to use the. ukca marking deadline for medical devices & ivds. What will the new uk regulation look like? deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. Ukca mark will be mandatory for. The uk. Ukca Marking Medical Devices Deadline.
From www.qualio.com
UKCA marking pathway guide Ukca Marking Medical Devices Deadline the deadline for when businesses need to use the ukca mark has been extended. Ukca mark will be mandatory for. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca. Ukca Marking Medical Devices Deadline.
From mavink.com
Ukca Marking Png Ukca Marking Medical Devices Deadline The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. You can continue to use the. but significant changes are planned. . Ukca Marking Medical Devices Deadline.
From www.exoltech.net
Know more about UKCA marking for Medical Devices Ukca Marking Medical Devices Deadline You can continue to use the. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. but significant changes are planned. a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. the deadline for when businesses need. Ukca Marking Medical Devices Deadline.
From casusconsulting.com
UKCA Marking Guide Medical Devices & IVDs (2024) Casus Consulting Ukca Marking Medical Devices Deadline Ukca mark will be mandatory for. You can continue to use the. a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. what’s the latest timetable for implementing ukca markings for medical devices? medical devices placed on the great britain market must have a. Ukca Marking Medical Devices Deadline.
From operonstrategist.com
UKCA Marking & Certification For Medical Devices (Update) Operon Ukca Marking Medical Devices Deadline what’s the latest timetable for implementing ukca markings for medical devices? ukca marking deadline for medical devices & ivds. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. the deadline for when businesses need to use the ukca mark has been extended. but significant changes are planned. Ukca. Ukca Marking Medical Devices Deadline.
From operonstrategist.com
UKCA Compliance A StepbyStep Guide for Medical Device Manufacturers Ukca Marking Medical Devices Deadline medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. You can continue to use the. a the gb market will accept ce certificates until 30. Ukca Marking Medical Devices Deadline.
From www.youtube.com
How to Get UKCA Marking as per UK MDR 2002 for Class 1 Medical Devices Ukca Marking Medical Devices Deadline You can continue to use the. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. Ukca mark will be mandatory for. what’s the latest timetable for implementing ukca markings for medical devices? the deadline for when businesses need to use the ukca mark has. Ukca Marking Medical Devices Deadline.
From international.cliniexperts.com
Medical devices conformity assessment and the UKCA mark CliniExperts Ukca Marking Medical Devices Deadline deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. What will the new uk regulation look like? the deadline for when businesses need to use the ukca mark has been extended. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device. Ukca Marking Medical Devices Deadline.
From www.qualio.com
UKCA marking pathway guide Ukca Marking Medical Devices Deadline what’s the latest timetable for implementing ukca markings for medical devices? a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. the deadline for when businesses need to use the ukca mark has been extended. What will the new uk regulation look like? You. Ukca Marking Medical Devices Deadline.
From rotechmachines.com
UKCA mark deadline fast approaching are you ready? Rotech Machines Ukca Marking Medical Devices Deadline What will the new uk regulation look like? ukca marking deadline for medical devices & ivds. You can continue to use the. The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. the deadline for when businesses need to use the ukca mark has been. Ukca Marking Medical Devices Deadline.
From www.pib-riskmanagement.co.uk
UKCA PRODUCT MARKING DEADLINE EXTENDED PIB Risk Management Ukca Marking Medical Devices Deadline what’s the latest timetable for implementing ukca markings for medical devices? The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. ukca marking deadline for medical devices & ivds. the deadline for when businesses need to use the ukca mark has been extended. What. Ukca Marking Medical Devices Deadline.
From www.fobalaser.com
UKCA marking on medical devices New regulations Ukca Marking Medical Devices Deadline The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. but significant changes are planned. ukca marking deadline for medical devices & ivds. What will the new uk regulation look like? medical devices placed on the great britain market must have a ukca marking. Ukca Marking Medical Devices Deadline.
From www.greensofttech.com
UKCA Mark Deadline Extended 2 Yrs Environmental Compliance GreenSoft Ukca Marking Medical Devices Deadline what’s the latest timetable for implementing ukca markings for medical devices? deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. Ukca mark will be mandatory for. but significant changes are planned. The uk government's website contains lots of information about the consultation process and the potential shape of future medical. Ukca Marking Medical Devices Deadline.
From www.qualio.com
UKCA marking pathway guide Ukca Marking Medical Devices Deadline deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. a the gb market will accept ce certificates until 30 june 2025 and, for certain medical devices, beyond this date if the requirements. but significant changes are planned. what’s the latest timetable for implementing ukca markings for medical devices? . Ukca Marking Medical Devices Deadline.
From www.medicalplasticsnews.com
Making your CEmarked device legal to sell in the UK Medical Plastics Ukca Marking Medical Devices Deadline The uk government's website contains lots of information about the consultation process and the potential shape of future medical device and ivdr regulation. the deadline for when businesses need to use the ukca mark has been extended. Ukca mark will be mandatory for. medical devices placed on the great britain market must have a ukca marking or a. Ukca Marking Medical Devices Deadline.
From www.i3cglobal.com
UKCA Marking Consultants For Medical Devices & IVDs Ukca Marking Medical Devices Deadline deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. what’s the latest timetable for implementing ukca markings for medical devices? medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. the deadline for when businesses need to use the ukca. Ukca Marking Medical Devices Deadline.